Drug Candidates
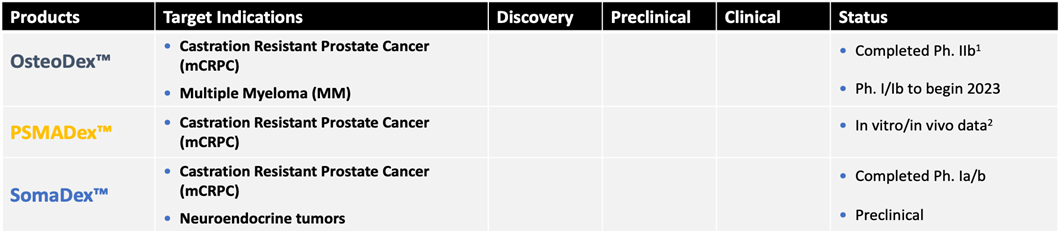
Dextech’s lead candidate ODX has been successfully developed through Phase 2b and is facing a Phase 3 study (mCRPC). Completed clinical studies show very low toxicity with few and mild side effects as well as a slowing effect on the disease (stable disease). One-third of the patients showed reduction in bone metastases. The Phase 2 study is published in Europe’s leading cancer journal, EJC (2023). The fact that the phase 2 study conducted with relatively few patients has been able to demonstrate about 1/3 of the reduction of bone metastases statistically indicates success in a phase 3 study with a larger patient base. The average response rate of cancer drugs is about 30%.
Due to several similarities between mCRPC and multiple myeloma, ODX is also being investigated for the treatment of multiple myeloma in a phase 1a study (2023).
Through the Company’s patented technology platform, GuaDex, 3 different drug candidates have been developed.
OsteoDex (ODX) is the Company’s lead candidate for the treatment of bone metastases from prostate cancer (mCRPC). ODX has completed a clinical phase 2b study (2020) and is facing clinical phase 3.
SomaDex, is natural somatostatin-14 linked to GuaDex with unique stability and maintained receptor binding ability. SomaDex should be used for the treatment of certain pituitary and neuroendocrine tumours and for the palliative treatment of mCRPC.
PSMA-Dx, is a PSMA specific tripeptide linked to GuaDex. Prostate-specific membrane antigen (PSMA) is found specifically on the cell membranes of CRPC cells. PSMA-Dex is a platform for target-specific treatment of CRPC. The platform allows you to choose the type of therapy (radionuclides or cytostatics). The interest in PSMA binding compounds is currently very high, especially after Novartis received market approval of its substance Pluvicto® (EMA, 2022) for the radioactive treatment of CRPC.
The company intends to continue the pre-clinical development of PSMA-Dex and explore early business opportunities. PSMA-Dex has global patent protection until 2037. Table 2 shows a summary of the candidates.
Table 2