Castration-resistant prostate cancer (CRPC)
CRPC is an incurable stage of prostate cancer in which approximately 90% develop metastases, mainly in the bones. Patients may have severe pain due to fractures, compression of the vertebrae, and other symptoms from the skeleton. In general, bone pain is the most common form of cancer-related pain and can be severe and disabling in a large proportion of patients, with a pronounced negative effect on quality of life and mobility. Experienced clinicians describe CRPC as a bone disease. Over time, existing treatment is ineffective and the patient dies from his disease. Median survival in CRPC is only about 1-2 years1.
Virtually all patients who die of prostate cancer, today about one in four patients, have castration-resistant disease. The main approved drugs for the treatment of castration-resistant prostate cancer are: docetaxel (Taxotere) and cabazitaxel (Jevtana), both of which are cytostatics, as well as abiraterone (Zytiga), enzalutamide (Xtandi) and Radium-223 (Xofigo). Abiraterone and enzalutamide are hormonally active (inhibitors/blockers) while Radium-223 binds to areas of the skeleton where secondary tumours (metastases) are located and emits a local radioactive radiation effect. These drugs have been shown to slow down tumor disease in most patients and prolong survival by about 2.5 – 5 months.
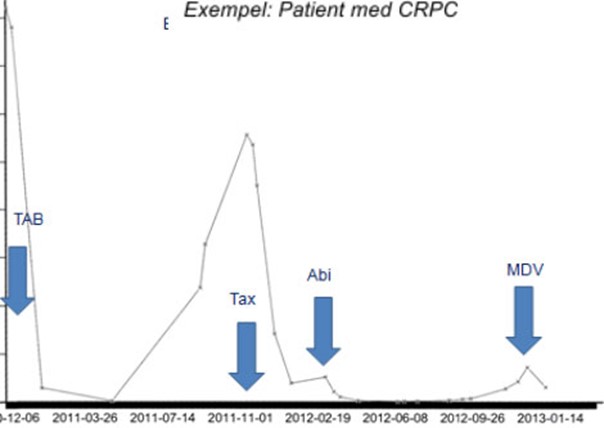
All of them have more or less serious side effects and the patient’s individual status determines which treatment can be used. All treatment of CRPC patients aims to be disease-slowing and palliative, where the treatment can, at best, prolong the life of the patient. Each of these drugs has a relatively short duration of action, as the disease becomes resistant to the drugs after a limited time and thus needs to be replaced by one of the other drugs (Figure 7). Provided that new drugs reach the market, CRPC could – at best – become a chronic disease. There is still no cure in sight.
Figure 7: y-axis = PSA microg/L, x-axis = time. The image shows a typical CRPC patient and the course of the disease. The Y-axis describes the cancer activity, PSA, in a patient where medication (blue arrows) reduces cancer activity but where the patient develops resistance to the medication after a period of time, whereupon PSA increases again. The patient is therefore given a new medicine and the cycle is repeated until no alternative medicines are available.
Against this background, DexTech is developing a complementary rather than a competing drug. The CRPC market is expected to continue to grow in the future due to an increasingly aging population. There is a great need for new drugs that can prolong life while maintaining a relatively high quality of life. OsteoDex has the potential to become an additional drug that can affect the prognosis, i.e. survival time with acceptable quality of life for patients with CRPC.
1 Kirby, M. , Characterising the castration-resistant prostate cancer population: a systematic review, The International Journal of Clinical Practice.